

Published on Nov 10, 2025
A Capitol Forum investigation has found that Linde Gas (LIN) appears to have been aware of a fatal flaw with its NOxBOXi nitric oxide therapy device for newborns for several years but continued to market the device to hospitals and represent to the Food and Drug Administration that it was safe and effective.
At issue is a compatibility problem with ventilators such as the Bunnell LifePulse High Frequency Jet Ventilator (HFJV) that produces wild swings in the dose of nitric oxide that neonatal patients receive from the NOxBOXi device.
“This was a known problem for the several years, since at least 2021,” a former Linde clinical sales representative, who asked to remain anonymous, told The Capitol Forum. “Ethically, it was becoming a challenge for me to interface with these hospitals and sell it as safe.”
Another former clinical sales representative who also asked to remain anonymous told The Capitol Forum that they were similarly concerned about selling a product they knew to be unsafe, saying “I was told just to continue to sell it as if there was no problem. I had to keep saying, ‘Everything is fine! There is a fix coming!’ to customers, but the problem was going on for years and they had no solution.”
According to the former clinical sales representatives, Linde did attempt to fix the issue in 2021 and 2022 by retrofitting devices in the field with additional circuits, but it was not effective.
“The real fix would have been to pull it off the market entirely,” the second former clinical sales representatives opined.
Linde Gas did not respond to multiple requests for comment for this article.
Incompatibility with only HFJV in country produced wild dose swings for neonatal patients. Inhaled nitric oxide (iNO) therapy, like that provided by the NOxBOXi, helps treat respiratory failure in neonates by relaxing and expanding the blood vessels in the lungs, which improves blood flow and oxygen delivery. The process is delicate, however, and a rapid increase or decrease in nitric oxide levels can cause life-threatening complications.
Currently, the Bunnell LifePulse ventilator is the only FDA approved HFJV available in the USA. Unlike traditional ventilators that mimic a typical, slower breath, an HFJV gently moves small amounts of air into and out of the lungs at very high rates, allowing for proper gas exchange while using as little pressure as possible to protect neonatal lungs.
“The NOxBOXi had a lot of trouble getting into rhythm with the Bunnell jet ventilator, keeping up with the fast pulses of the ventilator,” the first former clinical sales representatives explained. “Hospitals were having these huge dose swings, and respiratory therapists were calling us all the time, you could hear the alarms going off in the background of the call.”
“Everyone was well aware of what was going on. They were aware, they just were kind of at a loss about what to do about it or how to fix it,” the former clinical sales representative added.
HFJVs are typically only used for neonatal and pediatric patients who have severe respiratory failure or underdeveloped or injured lungs, which made the NOxBOXi’s problems all the more concerning to the former employees.
“That’s what really made this so hard, because jets are reserved for really sick kids,” the second clinical sales representatives said. “It is really life or death once a patient goes on the jet, and doctors and parents are going into it with a compromised device.”
Respiratory therapists have been voicing concerns about the NOxBOXi’s compatibility with jet ventilation in online forums as well. In a Reddit discussion from four years ago, respiratory therapists were complaining about wild swings in nitric oxide dosages, with one therapist saying that “we’ve contacted the company many, many times and they don’t seem to have a solid fix either.”
“The NoxBox is a ginormous hunk of dog shit,” another user posted. “I’ve never had so many problems with a nitric unit.”
In May of 2023, a healthcare provider reported this exact problem to the FDA. According to the report, a NOxBOXi “proceeded to completely stop working and stopped delivering nitric to this very critically ill patient… patient then started decompensating and bagging was required.”
“The patient was connected to a Bunnell HFJV high frequency jet ventilator. The NOxBOX [nitric oxide] delivery was set to 20 ppm [parts per million], but was fluctuating wildly anywhere from 76ppm to 6ppm,” the report states.
When this same problem with a Bunnell HFJV was reported again in March of 2024, the issue was clearly well known enough at Linde that the company appended the report with text noting that “dose fluctuations while using the Bunnell LifePulse ventilator using high frequency jet ventilation is a known possible occurrencewith the NOxBOXi device” (emphasis added).
In fact, a recent FDA inspection of Linde, first reported by The Capitol Forum, noted that the company had received over 50 complaints since January 2024 that identified “dose fluctuations as ‘potentially serious’ with HFJV use,” indicating that the company likely had data on the issue. Linde, however, “failed to consistently recognize the pattern of essential function failures and adequately investigate the clinical significance,” the FDA investigator observed.
Unfortunately, Linde Gas appears to have done little to correct or address this problem, and in March of this year a neonatal patient on a LifePulse ventilator died after experiencing dose fluctuations from two different NOxBOXi devices while on an HFJV. The recent FDA inspection noted at least two other infant deaths and several injuries that may have been caused by the NOxBOXi and Linde’s poor oversight of the device.
“Your firm’s repeated failure to implement timely and effective corrections for known safety issues poses ongoing risks to the neonatal population that depends on the NOxBOXi for life-sustaining therapy,” the FDA inspector wrote.
The risk to newborns is indeed ongoing, as the FDA inspector wrote. Last month, two reports of dose fluctuations with jet ventilators were submitted to the FDA; luckily, both NOxBOXi units were switched out and neither neonatal patient was harmed.
The FDA’s concern regarding Linde’s failure to recognize and address problems with the NOxBOXi stretch back to at least 2023.
According to an inspection from that year obtained by The Capitol Forum through a Freedom of Information Act request, the FDA observed that the company was failing “to investigate and identify the actions needed to correct or prevent reoccurring quality problems identified by your firm’s quality records, service records and/or complaint records.” That problem was not corrected by the time the FDA inspected the company again in 2025.
Linde appears to have been aware of wild dose swings with other types of ventilators. The Bunnell LifePulse was not the only ventilator that the NOxBOXi was having compatibility problems with, and Linde was not the only organization aware of the issue.
In March of 2024, a team of doctors in the Division of Neonatology at the Baylor College of Medicine published a paper titled “NOxBOXi fluctuations delivering inhaled nitric oxide via high‐frequency oscillator: A conundrum and a hypothesis.”
According to the paper, a one-day-old critically ill neonate was receiving iNO therapy when the “NOxBOXi screen kept fluctuating wildly by ±10 ppm of the set value of 20 ppm dialed in to deliver to the patient.” The patient appears to have been using a high frequency oscillating ventilator (HFOV) manufactured by Zoll, rather than Bunnell’s HFJV.
“Concerned about the potential effects of the actual delivery of iNO to the patient, NOxBOXi support line was called and upon inquiry we were advised that these fluctuations are known anomalies that have been noted on occasions when HFOV is at Hz of 10,” the researchers wrote (emphasis added).
The former sales representatives confirmed that there were known compatibility problems with oscillating ventilators, but they were not as prevalent as those with jet ventilators.
Baylor College of Medicine declined an interview regarding the paper and any other NOxBOXi incidents for this article.
Linde tells FDA that problematic ventilators are compatible with NOxBOXi. As previously noted, Linde informed users experiencing problems with the Bunnell and Zoll ventilators that dose fluctuations were “known” problems in March of 2024.
However, just a month later, Linde submitted an updated 510(k) application for the NOxBOXi to the FDA to expand the number of compatible ventilators with the device.
While Bunnell ventilators have been on the list of compatible ventilators for years, the updated application specifically adds ventilators manufactured by Zoll to the list:
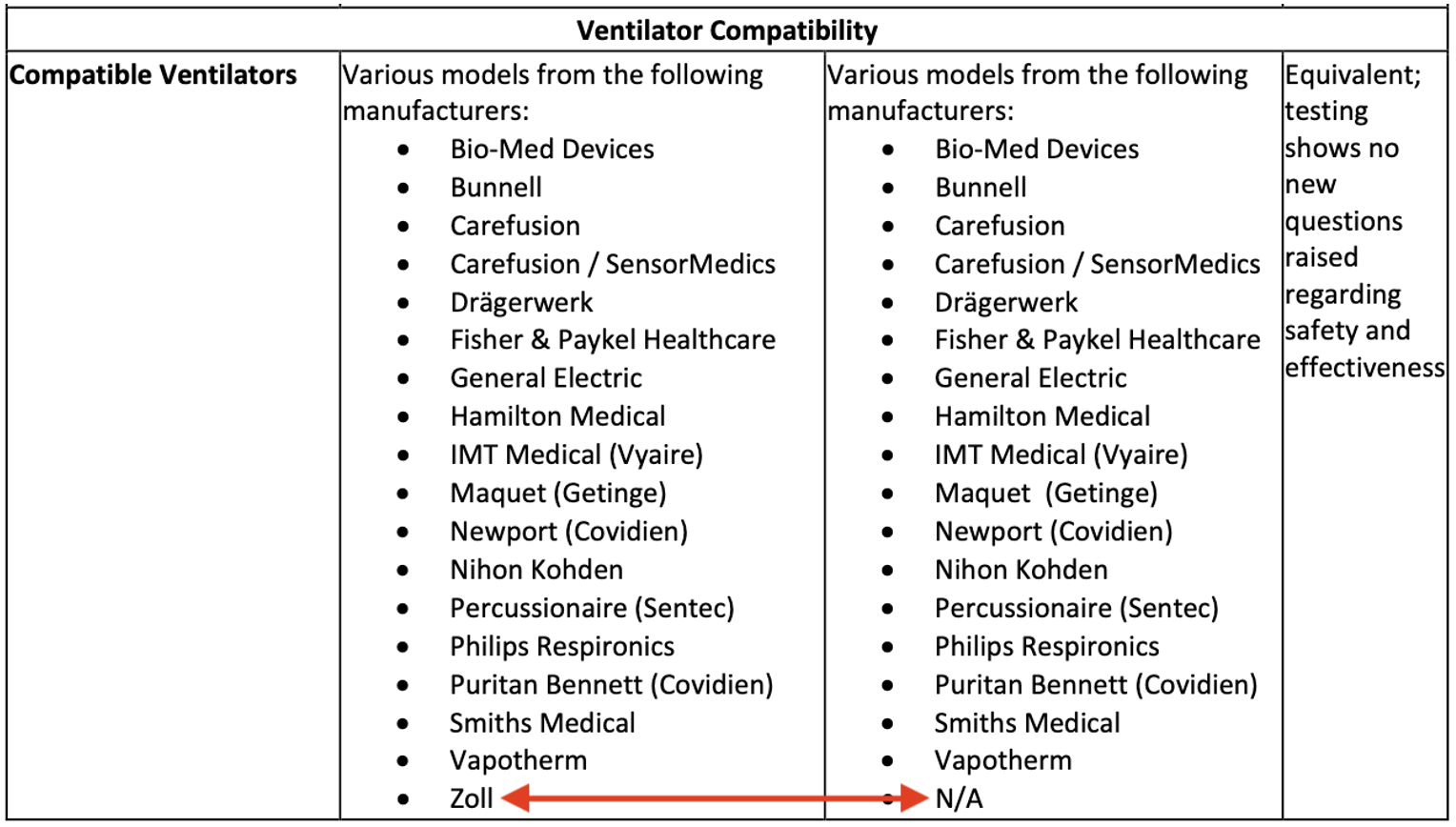
Source: NOxBOXi Nitric Oxide Delivery System 510(k) Application for FDA Premarket Approval
by Linde Gas & Equipment (K23325), Approved May 23, 2024
Note: Updated List of Compatible Ventilators Is on the Left
In the application, which the FDA approved a few weeks later, Linde represented that “the above-described non-clinical data support the substantial equivalence of the device and the compatibility with additional ventilators. The NOxBOXi passed all testing and no different questions of safety or effectiveness were raised.”
Marc Sanchez, a lawyer who specializes in FDA law, told The Capitol Forum that the representation of the Zoll and Bunnell ventilators as safe and compatible to the FDA was “likely a material omission.”
The sequence of events, Sanchez said, “would certainly prompt questions during an enforcement review. There are definitely enough questions for the FDA to pursue if it decides to do so.”
“A 510(k) must include a signed certification that all data are truthful and accurate and that no material fact has been omitted. False, incomplete, or misleading content can invalidate the certification and independently trigger enforcement exposure,” he explained. “Based on the above, the FDA can pursue recalls, Warning Letters, seizures, injunctions, civil money penalties, or require a new submission.”
Software “fix” only silenced low or high dose alarm. The 510(k) application Linde submitted in 2024 also included an additional change to the NOxBOXi. According to the application, the device was being updated to include “an additional optional software mode which disables the ‘Vent Flow Idle’ alarm to reduce this alarm which may not be necessary and is considered a ‘nuisance’ alarm in certain situations.”
That software change disabled the alarm that would ring when the nitric oxide concentrations became either too high or too low but did not actually correct the underlying problem of the dose swings itself.
“The software fix created an additional HFJV mode for the NOxBOXi, but all the mode did was silence the alarms and didn’t change the dose fluctuations,” the first former clinical sales representative explained. “All of the alarms were creating anxiety for respiratory therapists, and the update was promoted in the field as, ‘This is a software update and will help when it’s in HFJV mode,’ but it was a lot of smoke and mirrors. The alarm might not be going off, but the respiratory therapist could still see those fluctuations on the screen.”
This software update would eventually come under scrutiny by the FDA in its recent inspection of Linde, with the agency observing that the company had silenced the alarms without weighing the risk of the ongoing dosage fluctuations on infants.
According to the inspection report, “software modification to suppress ‘Vent Flow Idle’ alarms in HFJV mode were implemented on 5/28/2024 without adequately evaluating the impact the change has on reducing clinician awareness of potential risks that may cause under dosing of NO or toxic over exposure to NO2 in critically ill neonates due to the known HFJV software incompatibility causing dose fluctuations during therapy. This change appears designed to accommodate software incompatibility with the HFJV ventilators.”
A review of product recall alerts for the NOxBOXi indicates that Linde did not issue any recalls or safety notices to customers regarding the dose fluctuations, despite admitting knowledge of the problems as early as March of 2024.
Only recently did the company issue a recall for the dose fluctuation issue when the NOxBOXi is used with the Brunell HFJV and other ventilators, and that was only after the FDA’s inspection.
However, Linde does not appear to have a ready fix, as the recall states the company is “in the process of developing a new software version that will include a modification to the software to resolve this issue, which will be released pending required regulatory review and approval.”
Hospitals cancelled contracts with Linde over dose fluctuations; company required NDAs to cancel contract. According to the former clinical sales representatives, some hospitals either cancelled or attempted to cancel their contracts with Linde over the wild dose fluctuations caused by the NOxBOxi.
“This problem was starting to become well known in NICUs and that it wasn’t going away,” the first former clinical sales representative said. “I had one hospital that basically said, ‘This is a breach of contract because your device is not working as indicated.’”
Several of those hospitals, the clinical sales representative said, were able to get out of their contracts, and, in at least one instance, Linde required a hospital to sign a nondisclosure agreement promising not to disparage the company in order to be released from the contract.
The Capitol Forum reached out to several hospitals that had either reported problems with the NOxBOXi to the FDA or had been released from their contract. All of them either declined to speak with The Capitol Forumor did not respond to inquiries.
According to the former clinical sales representatives, the compatibility problems were well known enough in the industry that competitors, such as VERO Biotech and Mallinckrodt, began to use it as a sales tactic when selling against the NOxBOXi.
“Vero and Mallinckrodt would come in and say, ‘Look, we know you use jet ventilators. The NOxBOXi has this known problem with wild dose swings and we don’t,’” the first former clinical sales representative said.